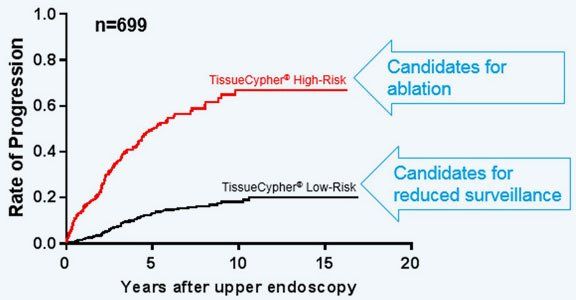
Pooled analysis of TissueCypher performance in five independent clinical validation studies.
TissueCypher®
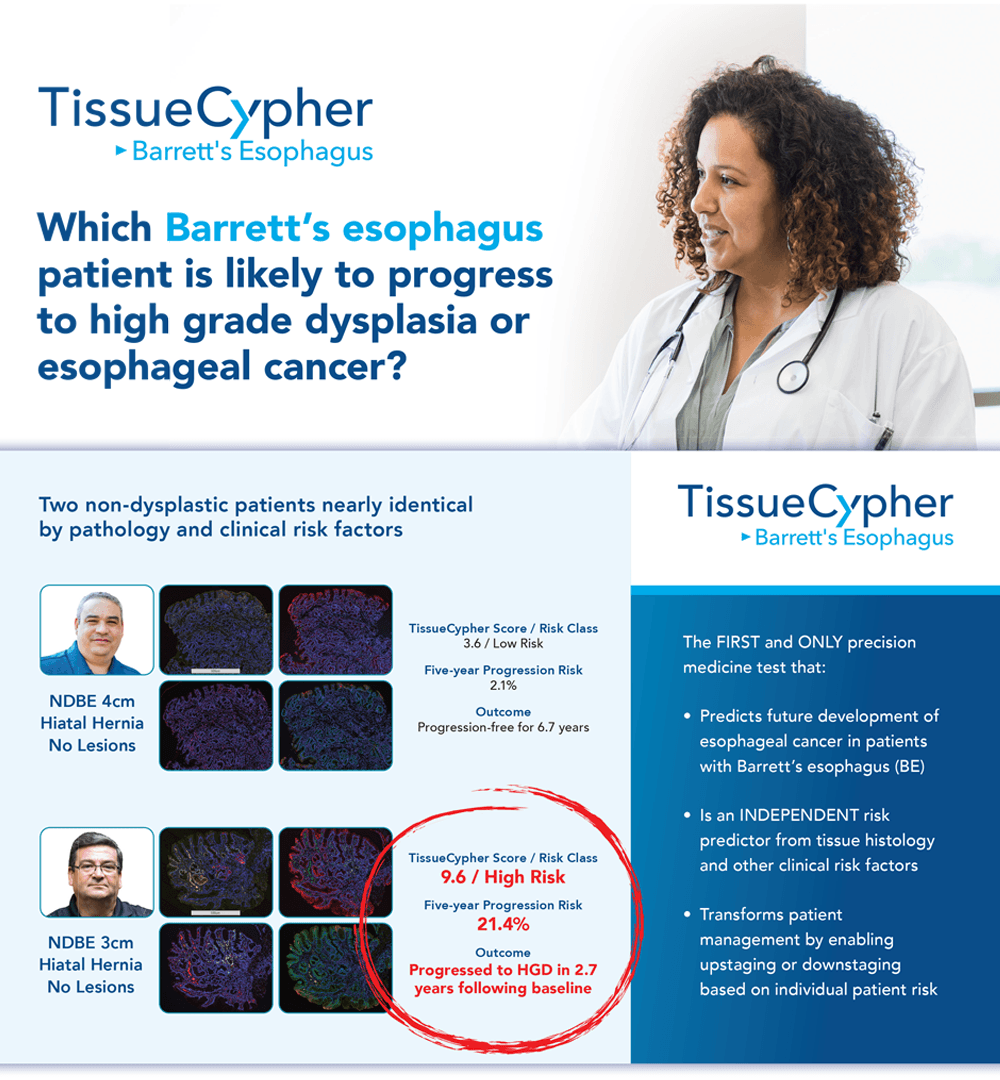
TissueCypher® Barrett’s Esophagus Assay is the first and only precision medicine test that can predict the future development of esophageal cancer in patients with Barrett’s esophagus and is an independent risk predictor from tissue histology and other clinical risk factors. The test is intended for patients with a confirmed diagnosis of Barrett’s esophagus graded non-dysplastic, indefinite for dysplasia or low-grade dysplasia, and provides a five-year risk of progression to high-grade dysplasia or esophageal adenocarcinoma.
New Study
Pooled Analysis
Study confirms an 18-fold higher risk of progression for non-dysplastic patients receiving a high-risk vs low-risk TissueCypher score.
What’s the best method for identifying a non-dysplastic Barrett's esophagus (NDBE) patients who is at high risk of progression to high-grade dysplasia (HGD) or esophageal cancer. TissueCypher® Barrett’s Esophagus Test can risk stratify NDBE patients, according to findings in a new study by lead author Prasad G. Iyer, M.D., M.Sc., professor of medicine in the Barrett’s Esophagus Unit of the Division of Gastroenterology and Hepatology at the Mayo Clinic, Rochester, Minn.

AGA Clinical Practice Update
Barrett’s esophagus screening and surveillance
Great news! The AGA recently published their latest Clinical Practice Update on Surveillance and Screening in Barrett’s Esophagus. A new Best Practice Advice statement (#9) highlights the use of TissueCypher to risk stratify non-dysplastic patients. The article cites evidence that a high-risk score for non-dysplastic patients has a progression risk similar to confirmed low-grade dysplasia (6.9%) and has been shown to be a strong independent predictor for progression to HGD/EAC (OR 14.2, 95% CI, 5-39, p<0.001).
Castle Biosciences
tissuecypher.com
Ian Fladoos
ifladoos@castlebiosciences.com
(971) 200-0437
Connect With Us on Social Media