

Powered Endoscopic
Debridement (PED)
for Necrosectomy
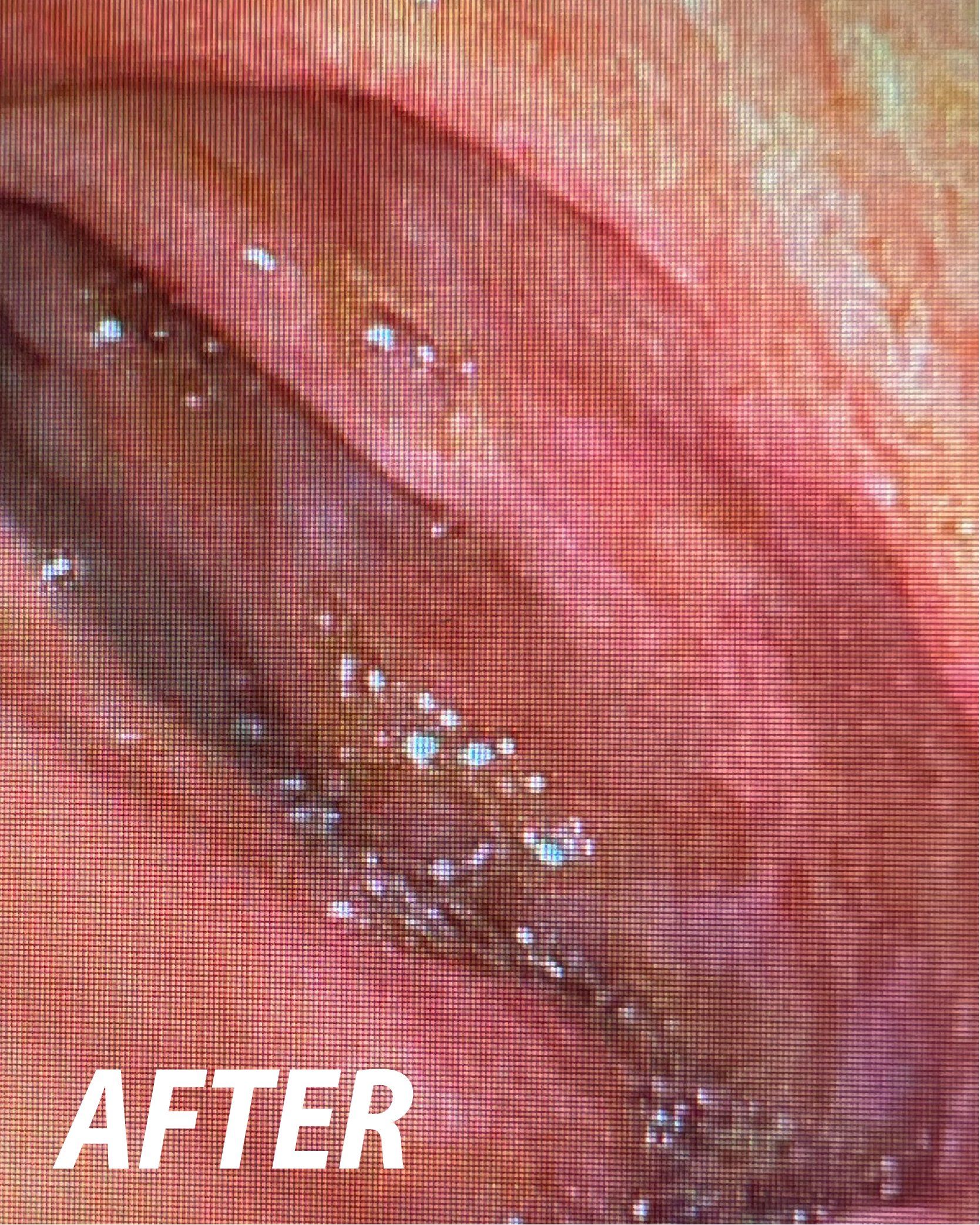
FDA De Novo – US: The EndoRotor® device is indicated to resect and remove necrotic tissue in symptomatic walled off pancreatic necrosis /walled off necrosis (WOPN/WON) after having undergone endoscopic ultrasound (EUS) guided drainage.
Endoscopic Powered
Resection (EPR)
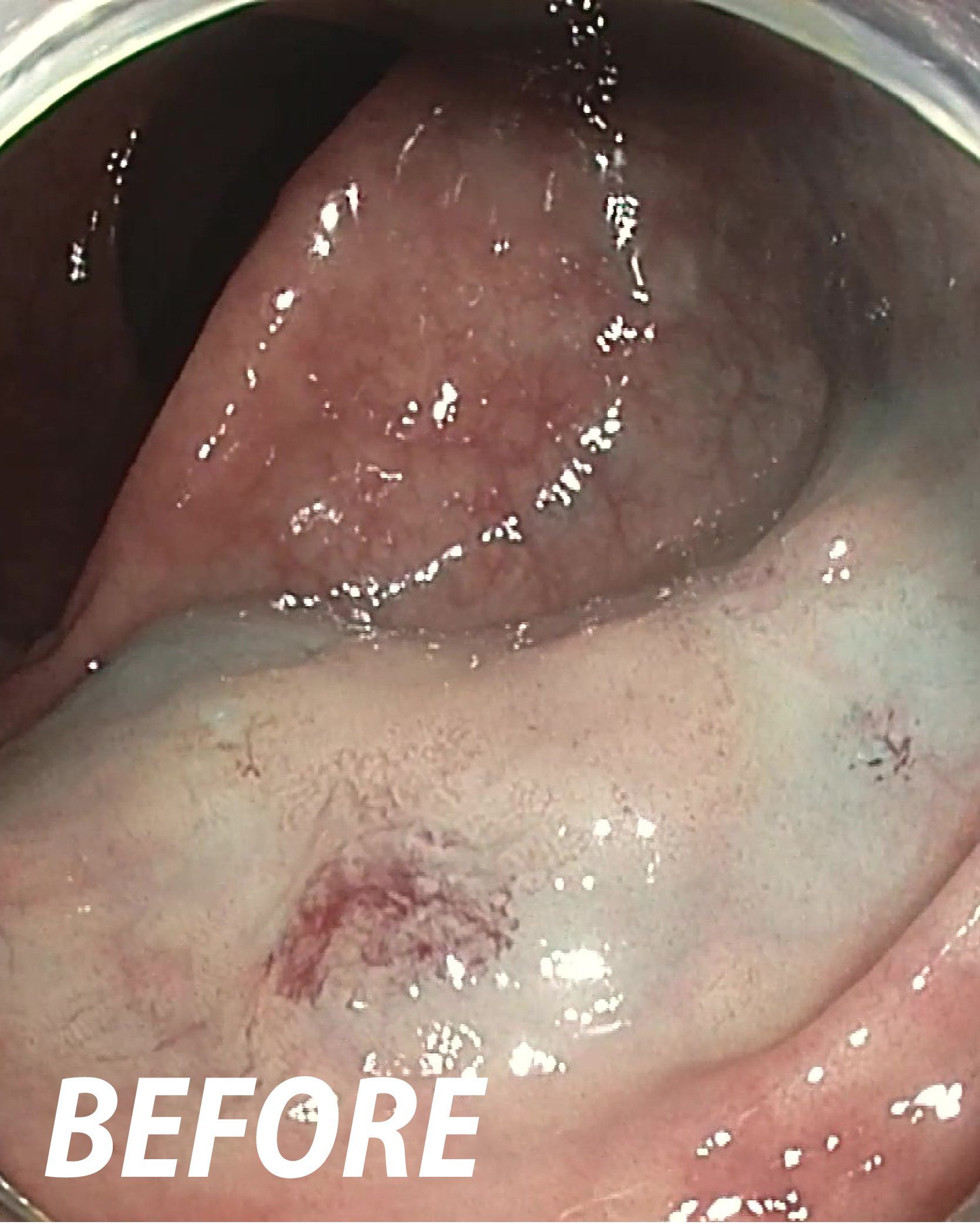
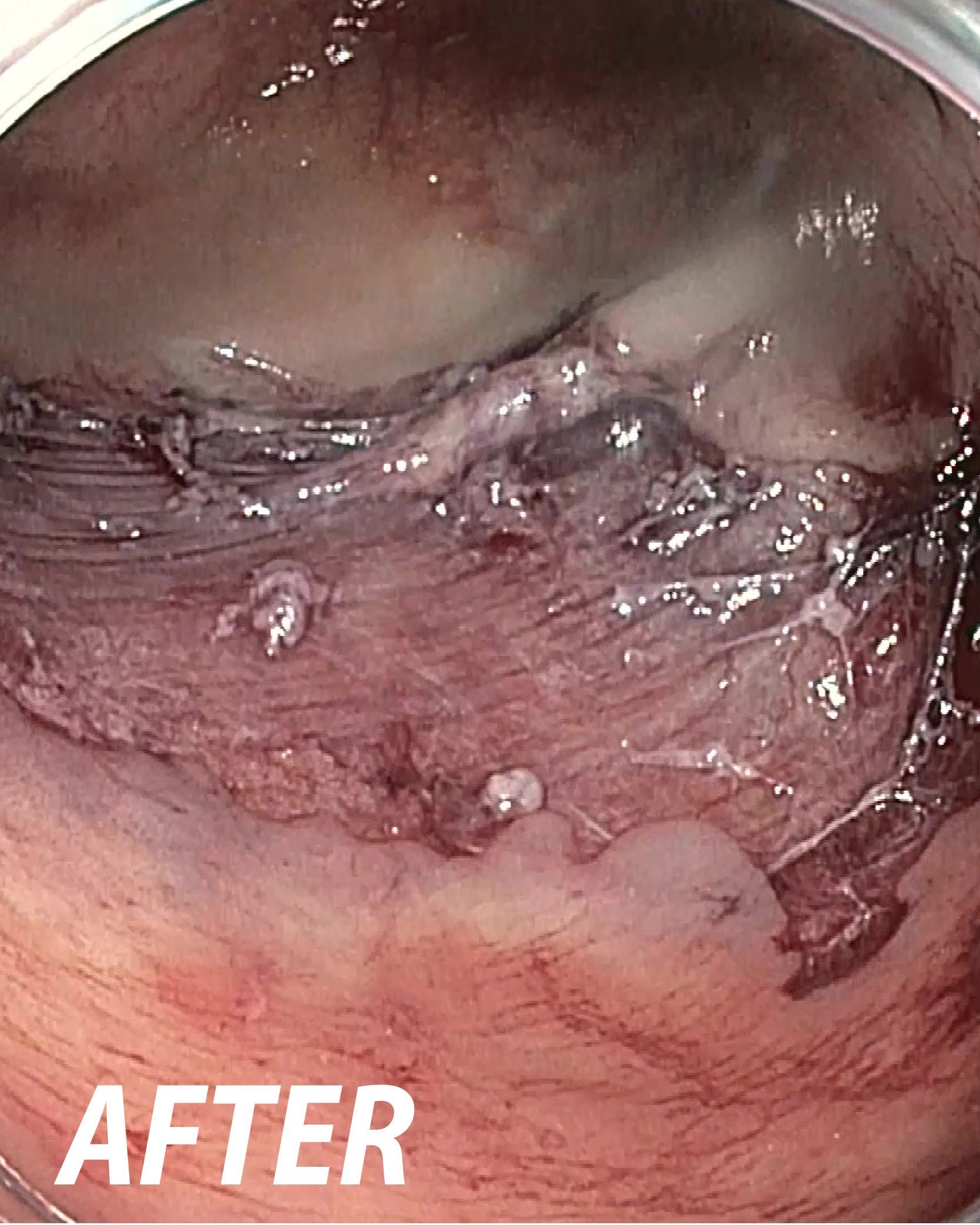
for Mucosectomy
FDA 510(k) – US: The EndoRotor® is intended for use in endoscopic procedures by a trained gastroenterologist to resect and remove tissue, not intended for biopsy, of the gastrointestinal (GI) system including post-endoscopic mucosal resection (EMR) tissue persistence with a scarred base and residual tissue from the peripheral margins following EMR.

Interscope
interscopemed.com
Justin Key
justin.key@interscopemed.com
720-745-2401
Connect With Us on Social Media

Slide title
Write your caption hereButton